Abstract
Introduction: Peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) is the most common PTCL subtype, accounting for approximately 25% of all PTCL cases. The median age at diagnosis is 60 years and is more prevalent among males. Currently, most epidemiological data on T- and NK-cell lymphomas come from developed countries. Thus, very few data are available regarding this condition in Latin American countries, especially in those aged 60 years and older. The Latin American Group of Lymphoproliferative Disorders (GELL) has previously reported on the impact of serum albumin level in survival outcomes in PTCL-NOS. Therefore, we aim to evaluate the clinical, demographic, and outcome patterns of elderly patients with PTCL-NOS.
Methods: We conducted an observational, retrospective study of newly diagnosed PTCL-NOS between January 2000 and December 2020. Patient must be aged 60 years and older. All clinical data were retrieved from clinical records at the different participating institutions. The study outcomes were overall survival (OS) and progression-free survival (PFS). The International Prognostic Index (IPI), the Prognostic Index for T-cell lymphoma (PIT), and the NCCN IPI scores were used for risk stratification. Kaplan-Meier and log-rank test were used for survival analysis. Univariate and multivariate Cox regression analysis were used to estimate hazard ratios (HR) with a 95% confidence interval (CI). Outcomes with a p-value <0.05 were considered statistically significant.
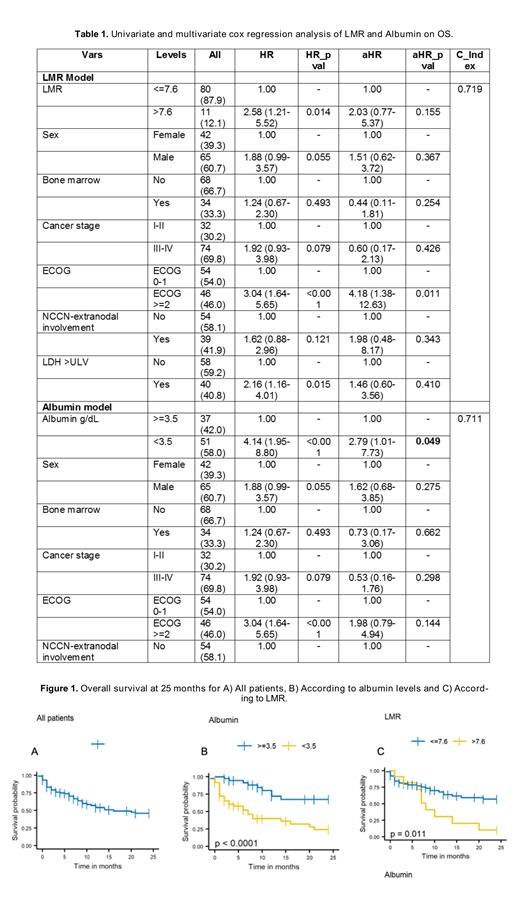
Results: A total of 107 patients were included; median age at diagnosis was 72 years (range 60-92), 61% were male, 46% had an ECOG ≥2, 70% had stage III-IV, and 15% had more than one extranodal site. The IPI, PIT and NCCN-IPI scores stratified 51%, 69%, and 74% of patients as high- / high-intermediate risk, respectively. Seventy-four (69%) patients received first-line systemic therapy. Most patients (53%) were managed with CHOP/CHOP-like regimen, followed by CHOEP (19%). The overall response (OR) rate to first-line therapy was 61% (complete response, CR 41%). Salvage therapy was given to only 9 patients; the OR rate was 44% (CR 11%). With a median follow-up of 29 months (95% CI 13-45 months), the 2-year OS was 46% (95% CI 36-59), with median survival time of 16 months (95% CI 10-NR). In the multivariate analysis, patients with serum albumin levels <3.5 g/dL had median survival of 7 months (95% CI 3-20) and 2-year OS rate of 24% (95% CI 13-46%), while patients with albumin ≥3.5 g/dL did not reach the median survival time and had a 2-yOS rate of 67% (95% CI 36-65%) (p<0.0001) (Table 1, Figure 1). Patients with a lymphocyte-to-monocyte ratio (LMR) >7.6 had a median OS of 8 months (95% CI 6-NR) and a 2-year OS of 10% (95% CI 2-65), while patients with a LMR ≤7.6 did not reach the median OS and had a 2-year OS of 57% (95% CI 45-72) (p = 0.0108) (Table 1, Figure 1).
Conclusions: This large cohort of Latin American PTCL-NOS patients aged ≥60 years showed overall response rates comparable to previously published data. However, survival rates were shorter compared to those reported in developed countries. In this study, we again confirmed low serum albumin level (<3.5 g/dL) as an adverse prognostic factor for OS in PTCL-NOS, and independent from the IPI, PIT and NCCN-IP scores. Additionally, we describe for first time in a Latin American cohort the impact of the LMR on survival, demonstrating poor outcomes in those with LMR >7.6. We are currently validating our findings in a prospective cohort of Latin American PTCL patients and to improve clinical decision-making in those deemed at high-risk for early mortality.
Otero: Astra Zeneca: Current Employment. Castillo: Abbvie: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Janssen: Consultancy; Roche: Consultancy; TG Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal